Essential Guide to Change Control in Pharmaceutical Industry
Summary – Change Control in Pharma
In the ever-evolving world of pharmaceuticals, ensuring product quality, efficacy, and safety is paramount. Change is inevitable; whether driven by technological advancements, regulatory mandates, or the continuous pursuit of operational excellence.
Yet, with every alteration, comes the vital responsibility of managing and documenting these changes meticulously. Welcome to the indispensable realm of change control—a structured approach that stands as the backbone of the pharmaceutical industry.
This guide delves deep into the intricacies of change control, its significance, methodologies, and best practices that industry professionals must master to uphold the highest standards of drug development and production. Join us as we journey through the corridors of this critical discipline, ensuring that every shift in the pharmaceutical landscape is navigated with precision and accountability.
In pharmaceuticals, change control is crucial for adjusting established procedures, systems, or products, guaranteeing quality medicine production in line with regulations. Temporary change control focuses on short-term deviations, which are documented for product integrity. Deviations, whether unplanned or intentional, are categorized as minor, major, or critical based on their impact, streamlining evaluation.
The change control process begins with identifying and justifying proposed changes, setting the stage for effective management. A preliminary assessment evaluates immediate impacts, regulatory concerns, and risks. This segues into a detailed review, ensuring alignment with quality, safety, efficacy, and regulatory standards. The decision, made by cross-functional change control boards, balances safety, compliance, feasibility, and operational impact.

Guide to Effective Change Control Management in Pharma
Effective communication threads through the entire process. It’s paramount to keep stakeholders informed and ensure all regulatory facets are addressed. Upon a change’s approval, the spotlight shifts to implementation. A comprehensive plan is crafted, essential documentation updated, and targeted training sessions conducted. This change, once initiated, must adhere strictly to established protocols. Verification, achieved through tests, inspections, and audits, is key to ensuring accurate execution.
In certain scenarios, validation becomes necessary, affirming the change’s lasting efficiency. As the process concludes, formal closure of the initiative is paired with post-implementation evaluations. Gathering stakeholder feedback is crucial at this juncture, offering insights that can refine future change management endeavors.
Lastly, visual tools like change control flowcharts and change control in pharma PPT amplify clarity. They are instrumental in demystifying the process, enhancing training, and solidifying communication and compliance. The overarching goal of change management in pharmaceuticals is clear: upholding regulatory standards, guaranteeing product quality, managing inherent risks, and most importantly, ensuring patient well-being.
If you’re ready to jump into the world of change control, make your way down to our Table of Contents.
|
DETAILED SECTION |
|
If you’re ready to delve deeper into the world of change control in pharma, proceed to our detailed sections below that provide a detailed overview. |
Table of Contents: Change Control Pharmaceutical Guide
Keep on scrolling down this page to read each section or click any link below to go directly to that section.
- Change Control Definition in Pharma
- What is Temporary Change Control in Pharma?
- Types of Change Control in Pharma
- Change Control Process in Pharma | Guidelines for Change Control in Pharmaceuticals
- Change Control Flowchart in Pharma
- Importance of Change Management in Pharmaceutical Industry
- Free Change Control in Pharmaceutical Industry PPT
- Conclusion | Change Control Guidelines in Pharma
- FAQ | Quick Overview of Change Control in Pharma
See Also: Ultimate Guide on Project Management Change Control
Change Control Definition in Pharma
Within the domain of change control in pharma, the change control definition in pharma illustrates a thorough approach to managing all alterations that might influence the pharmaceutical product’s quality.
The definition of change control in pharma refers to a systematic approach employed within the pharmaceutical industry to manage and document any modifications to established processes, systems, or products.
This ensures the consistent production of high-quality medicinal products, even in the face of alterations.

What is Change Control Definition in Pharma?
Additionally, change control definition in pharma emphasizes the importance of documenting and evaluating any proposed changes to ensure they do not adversely impact the quality, safety, or efficacy of the pharmaceutical product.
It is a critical component of quality management systems, aiming to mitigate risks associated with changes while ensuring compliance with regulatory standards.
With a clear change control definition in pharma outlined, let’s take a quick look at temporary change control in pharma so we can make a clear distinction between the two.
Do you need more information on change control pharmaceutical procedure in general? Please reach out and let us know.
Temporary Change Control in Pharma
Temporary change control in pharma represents a deviation from the usual process but with a clear end date.
Using the specific change control format in pharma, these temporary shifts are systematically documented, assessed, and approved.
The change control format in pharma ensures that any adjustments, even short-term ones, maintain product integrity and safety.
Temporary change control examples in pharma include using an alternative ingredient source due to sudden supply issues or adjusting equipment settings temporarily.
In all these temporary change control examples in pharma, it’s essential to assess the risks and potential impacts on the product.
It’s worth noting that temporary change control in pharma often intersects with “deviation and change control in pharma.”
What does deviation and change control in pharma refer to?
Deviation, by nature, might be unplanned, and thus the change control format in pharma provides a pathway to manage them.
When a deviation occurs, it’s documented and evaluated, and sometimes leads to a temporary change control in pharma while a permanent solution is sought.
The differentiation between deviation and change control in pharma becomes clear through example of change control in pharma, where deviations might be unexpected, while changes are planned adjustments.
Temporary change control in pharma, backed by a structured change control format in pharma, ensures deviations are managed effectively.
The numerous change control examples in pharma highlight the importance of this balance, ensuring both deviation and change control in pharma work cohesively to safeguard product quality.
Now that we know the difference, we can move to types of change control in pharma.
Related: Best Guide to ITIL Change Control Process with Free Templates
Types of Change Control in Pharmaceutical Industry
Change control management in pharmaceutical industry is integral to ensure product quality, efficacy, and safety.
In the intricate world of pharmaceutical manufacturing and drug development, any alteration to processes, equipment, or procedures can have a significant impact on the final product.
Recognizing the gravity of these changes, the pharmaceutical industry has established stringent guidelines for managing them. Central to this system is the concept of change control in pharma.

Change Control Pharmaceutical Types
The types of change control in pharma are especially critical because they determine the scope of the assessment, validation, and documentation required.
These modifications, based on severity, uncertainty, and importance, are categorized under distinct types of change control in pharmaceutical industry.
Types of change control in pharma often use a three-tier system:
- Minor Change
- Major Change
- Critical Change
In the sections below we’ll give you a quick overview of each of the types of change control in pharmaceutical industry.
1. Minor Change
Minor changes are those that have minimal potential to impact the product’s quality, efficacy, or safety.
Often seen as administrative or procedural tweaks, they may not directly influence the therapeutic attributes of the drug.
While these changes might seem inconsequential, they still warrant documentation due to the pharmaceutical change control.
The meticulousness of types of change control in pharma ensures that even these low-risk adjustments are recorded for transparency and traceability.
Do you have any questions about change control procedure in pharma? Don’t hesitate to reach out and let us know.
2. Major Change
These changes have a moderate to high potential to influence a product’s characteristics.
They often necessitate comprehensive assessments and might include alterations like changing a raw material supplier or tweaking a formulation component.
Given their potential implications, these changes require a thorough:
- review,
- validation, and
- possibly regulatory communication.
Within the spectrum of types of change control in pharma, major changes hold significant weight and are approached with heightened scrutiny.
3. Critical Change
Critical changes are the most profound and have the highest potential to alter the drug’s quality, safety, or efficacy.
They often come with a high degree of uncertainty and importance.
Critical changes might involve a complete overhaul of a production process, the introduction of new manufacturing technology, or a significant modification to the drug’s active ingredient.
Due to the potential risks and ramifications, the types of change control in pharma for critical changes demand:
- exhaustive testing,
- validation, and
- regulatory approval.
The rigorous change control pharmaceutical standards ensure that these changes are managed with the utmost care and caution.
Through the implementation of these types of change control in pharma, and with the guidelines dictating change control pharmaceutical industry, manufacturers maintain a dynamic yet controlled environment, ensuring that every product meets the highest standards.
Next, it’s time to get a good understanding of the change control pharmaceutical process.
Do you have any questions about guidelines for change control in pharmaceuticals? Please reach out and let us know. We’ll love to hear from you.
Change Control Process in Pharma | Guidelines for Change Control in Pharmaceuticals
The change control process in pharma is a formal, systematic approach to ensuring that any changes to processes, equipment, or materials do not adversely impact the quality, safety, or efficacy of drug products.
Effective change control procedure in pharma is essential to maintain the consistent production of high-quality drugs and to ensure regulatory compliance.
Here’s an overview of the change control process in pharma:
Stage 1 – Identification of Change
The change control process in pharma begins when a change is proposed.
The reasons for change initiation in the pharmaceutical sector can be multifaceted, and identifying the exact nature and reason for the change is crucial for proper change management in pharmaceutical industry.
This could be a result of:
- An equipment or facility upgrade
- Changes in raw material sources or suppliers
- Process improvements or modifications
- Regulatory or compliance requirements
During the identification phase of the change control procedure in pharma, it’s vital to document:
- the reason for the change,
- its potential scope, and
- any preliminary assessments or observations.
This documentation not only provides clarity for the team involved but also serves as a record for future audits or reviews.
In essence, the identification step of the change control pharmaceutical process is not merely about recognizing that a change is necessary, but understanding the ‘why’ behind it, and gauging the potential impact it may have on the broader production process and the final product.
Do you have any input on change control SOP in pharma or example of change control form in pharma that we haven’t mentioned? If so, don’t hesitate to get in touch.
Stage 2 – Justification for Change
The justification step is a pivotal checkpoint in the change control process in pharma.
Just as it’s imperative to identify a potential change, it’s equally crucial to provide a compelling reason for implementing it.
This step of the change control pharmaceutical procedure acts as a gatekeeper, ensuring that changes are not made hastily or without proper reasoning, thus maintaining the integrity and quality of pharmaceutical products.
Here’s a deeper look into the Justification for Change step of the change control process in pharma:
⋅ Purpose and Objective
Every change, irrespective of its magnitude, must have a clear purpose.
The objective might range from:
- improving efficiency,
- ensuring safety,
- maintaining regulatory compliance,
- enhancing product quality, or
- addressing other specific challenges.
By explicitly stating the purpose, stakeholders have a clear understanding of the end goal, ensuring alignment and focus throughout the change control pharmaceutical process.
⋅ Risk vs. Benefit Analysis
Every change comes with its inherent risks and benefits.
While the process of change control in pharmaceutical industry might offer advantages, it’s essential to understand any potential drawbacks, costs, or challenges.
A thorough risk vs. benefit analysis helps in determining if the benefits of the change outweigh the potential risks and costs associated.
This can include:
- financial implications,
- impacts on product quality,
- potential downtimes, or
- implications for re-validation.
⋅ Resource Assessment
Implementing change often requires resources – this could be in terms of manpower, finances, time, or technical expertise.
Justification step of the change control procedure in pharma should include an assessment of the required resources versus what’s available.
As an example of change control in pharma; if a process improvement requires a specialized piece of equipment that’s costly and not within the budget, the justification for such a change would need to be compelling.

Change Control Guidelines in Pharma
⋅ Impact on Stakeholders
Every change might have implications for various stakeholders, including production teams, quality control departments, regulatory bodies, and even the end consumers.
The justification step of the change control in pharmaceutical industry should take into account how these stakeholders might be affected, ensuring that their needs and concerns are addressed.
⋅ Alignment with Strategic Goals
It’s also important to see how the proposed change aligns with the organization’s strategic goals and objectives.
A good example of change control in pharma would be a scenario where a pharmaceutical company’s strategic intent is to speed up production without compromising quality – then any proposed change should ideally contribute to that goal.
⋅ Documented Evidence or Data
A robust justification often rests on concrete data or evidence.
This might be in the form of:
- research studies,
- benchmarking data,
- feedback from stakeholders, or
- other empirical evidence that supports the need for change.
⋅ Regulatory Considerations
In the pharma industry, regulatory compliance is paramount.
Therefore, any justification should also consider the regulatory implications of the change.
Example of change control in pharma; if a change helps in achieving compliance with a newly introduced regulation, then its justification becomes even stronger.
The justification for change isn’t just a formality but a comprehensive evaluation process.
It ensures that changes are well-thought-out, beneficial, and in alignment with both immediate needs and long-term goals.
Proper justification step of change control in pharmaceutical industry provides confidence to stakeholders and regulatory bodies that changes are made with due diligence and a commitment to maintaining the highest standards of quality and safety.
If you’re looking for change control in pharmaceutical industry PPT that summarizes the whole process in an easy to share document, read on!
We have a change control in pharma PPT waiting for you further down in this article.
Next, we’re moving on to Preliminary Assessment/Review stage.
Related: Everything You Need to Know About Stage Gate Model in OCM & Project Management
Stage 3 – Preliminary Assessment/Review
The preliminary assessment or review serves as the initial screening phase in the change control pharmaceutical process.
While the earlier steps focused on identification and justification, this phase of the pharmaceutical change control is centered around understanding the potential breadth and depth of the change’s implications.
A concise overview of the Preliminary Assessment/Review includes:
⋅ Immediate Impacts
At the outset, the assessment will quickly gauge if there are any direct consequences of the proposed change on product quality.
This means examining if the change might result in any variations in product specifications, stability, efficacy, or other quality attributes.
Example of change control may include a scenario where a change in a raw material might directly impact the formulation and, subsequently, the product’s bioavailability.
⋅ Regulatory Implications – Guidelines For Change Control In Pharmaceuticals
The pharma industry is tightly regulated, and any change, however minor it might seem, could have regulatory ramifications.
The preliminary assessment checks if the proposed change could potentially breach any existing regulatory standards or if it necessitates fresh regulatory approvals or notifications.
⋅ Potential Risks
This step of the change control pharmaceutical procedure includes a cursory glance at possible risks, even if they aren’t immediately evident.
Example of change control in pharma; introducing a new machine could pose risks not just in terms of operational efficiency but also regarding employee safety or cross-contamination.

Change Control Guidelines in Pharma
⋅ Scope of Change
One primary goal of this phase of the change control pharmaceutical procedure is to categorize the change based on its magnitude.
Is it minor, with limited impacts, or is it major, with broader consequences? Determining this helps in deciding the subsequent steps, especially in terms of validation or deeper risk assessment.
⋅ Need for Further Analysis
Depending on the initial findings, it might be evident that a more in-depth analysis or a full-fledged risk assessment is required.
For significant changes that have extensive implications, this step of the change control pharmaceutical procedure acts as a precursor to a more comprehensive evaluation.
⋅ Stakeholder Involvement
This review can also identify which internal or external stakeholders need to be involved as the change control pharmaceutical process progresses.
Different changes might require expertise from varied departments, be it R&D, quality assurance, production, or regulatory affairs.
⋅ Documentation
Even though this is a preliminary step, documentation is crucial.
Initial findings, observations, concerns, and decisions taken during this phase of the change control pharmaceutical process are recorded. This ensures transparency, aids in subsequent steps, and provides an audit trail.
To summarize what we discussed – Preliminary Assessment/Review step of the pharmaceutical change control acts as a filter.
It’s a checkpoint that ensures only warranted changes progress to the more resource-intensive evaluation stages.
By identifying potential impacts and risks at this early stage of the change control pharmaceutical process, companies can make informed decisions, ensuring that resources are allocated judiciously and that product quality and regulatory compliance are always at the forefront.
Want to have all of this info in compact view? Read on and get your change control in pharmaceutical industry PPT.
Moving into Detailed Evaluation next!
Stage 4 – Detailed Evaluation
If the change is approved for further consideration, a detailed evaluation is performed.
This step of the change control pharmaceutical process includes:
⋅ Determining the impact on the product’s quality, safety, and efficacy
Quality: Ensures the change doesn’t compromise the product’s established quality parameters like stability and potency.
Safety: Examines potential risks, such as increased contamination chances or new impurities.
Efficacy: Assesses if the change affects the drug’s therapeutic effect.
⋅ Reviewing the effect on current validation status
Evaluates if the change impacts previously validated processes and if re-validation is necessary. This encompasses equipment calibration and method validation checks.
⋅ Assessing potential regulatory implications
Regulatory Reporting: Determines if new reports to regulatory bodies are needed.
Regulatory Compliance: Checks alignment with current regulations and potential future challenges.
⋅ Evaluating the necessary resources and timeframe for implementing the change
Assesses manpower needs, costs (direct and indirect), technical resources, and establishes a realistic timeline for the change’s execution.
In essence, the Detailed Evaluation stage of the change control pharmaceutical procedure ensures that any proposed change doesn’t compromise the product’s safety, efficacy, or quality, aligns with regulations, and is efficiently managed in terms of resources and time.
Read More: Tips for Dealing with Resistance to Change in the Workplace
Stage 5 – Approval or Rejection
Based on the detailed evaluation, the change may be approved or rejected.
This decision is usually made by a change control board (CCB) or committee comprising:
- quality assurance,
- manufacturing,
- engineering,
- regulatory affairs,
- and other relevant departments.
Here’s a brief overview of this phase:
⋅ Change Control Board/Committee
A cross-functional team from key departments, including quality assurance, manufacturing, and regulatory affairs, ensures a comprehensive assessment of the change.
⋅ Decision Criteria
Factors considered include:
- Safety and Efficacy: The product’s safety and therapeutic effect must remain uncompromised.
- Regulatory Compliance: Alignment with all regulatory standards.
- Operational and Resource Feasibility: Can the change be implemented without disruption, given available resources?
⋅ Documentation
Every decision, whether approval or rejection, is documented, highlighting reasons and any associated conditions or feedback.
⋅ Feedback
In cases of rejection, feedback is provided to the proposer, indicating areas of concern or improvement.
⋅ Communication
The decision is relayed across the organization to keep all relevant departments informed and aligned.
Next, we’re moving to the Communication phase of change control management in pharmaceutical industry.
Don’t Miss: Adapting to Change in the Workplace | All You Need to Know
Stage 6 – Communication
Throughout the process, effective communication is essential in change control pharmaceutical process.
Stakeholders, including those in production, quality control, and regulatory affairs, must be informed of the change and its implications.
⋅ Broad Awareness
Changes need to be communicated across departments, from R&D to manufacturing and quality control, ensuring unified understanding.
⋅ Feedback Mechanism
Open channels enable diverse teams to share insights, concerns, or suggestions about the proposed change.

⋅ Stakeholder Engagement
Key stakeholders, like suppliers or distributors, must be informed if changes affect their roles or operations.
⋅ Regulatory Notification
For major changes, timely communication with regulatory authorities is critical to maintain compliance.
⋅ Documentation
All change-related communications should be documented for transparency, future reference, and audit preparedness.
By ensuring clear communication, the pharma industry can smoothly navigate changes, minimizing errors and maintaining product integrity.
Looking for a change control in pharmaceutical industry PPT to communicate this effortlessly to your team?
OCM Solution has got you covered! Find the change control in pharma PPT slideshow you can adjust to your needs.
Next stage, Implementation!
Do you have any questions or input on change control in pharmaceutical industry PPT or example of change control form in pharma? Just reach out and let us know.
Stage 7 – Implementation
When a change is approved, the implementation phase of the change control pharmaceutical process comes into play.
Properly executing this stage is crucial for ensuring the integrity of the change and maintaining the standards that the pharmaceutical industry demands.
In the pharmaceutical realm, the implementation of any approved change must adhere to strict protocols:
⋅ Development of a Detailed Plan
Using the change control SOP in pharma as a guide, a strategic plan for introducing the change is formulated. If re-validation is needed, this plan will incorporate it.
⋅ Document Updates
As part of rigorous change management in the pharmaceutical industry, affected SOPs, batch records, and related documents are revised. Utilizing a change management SOP template ensures consistency and alignment with best practices.
⋅ Training
Personnel impacted by the change undergo training as per the change control SOP in pharma to ensure flawless execution and understanding.
⋅ Execution
Implementing the change is a critical step. Here, the change management SOP template guides the process, underlining the significance of meticulous change management in the pharmaceutical industry.
In essence, the precise implementation of changes, guided by established change control in pharma SOP resources, upholds the stringent standards of the pharmaceutical sector.
With that summarized, let’s move on to Verification.
Popular Article: Complete Guide to Top Change Management Models, Frameworks, & Change Methodologies
Stage 8 – Verification
After the change has been implemented, it’s crucial to verify that it was executed correctly and that there are no unintended consequences.
This step of the change control pharmaceutical procedure might involve:
- Testing: Specific tests, possibly both qualitative and quantitative, are carried out to confirm that the change meets its intended objectives and that no detrimental effects on product quality or process efficiency have occurred.
- Inspections: Physical inspections may be necessary, especially when the change involves equipment, facilities, or tangible materials. This helps in ensuring that installations or modifications adhere to standards and specifications.
- Audits: An audit, which can be internal or external, reviews the documentation, processes, and outcomes related to the change. It ensures that the implementation aligns with the change control SOP, regulatory requirements, and industry best practices.
Through these verification activities, the pharmaceutical entity ensures that the change not only meets its intended purpose but also maintains the integrity of the product and process.
After verification comes Validation stage of the change control pharmaceutical procedure, comes Closure and Review.
Would you like to share example of change control form in pharma that was valuable to you? Just reach out and let us know.
Stage 9 – Closure and Review
The final step in the change control process is to formally conclude the initiative and reflect upon its long-term implications:
⋅ Formal Closure
After ensuring the change has been successfully implemented, verified, and all necessary documentation is in place, the change control request can be formally closed in the system or registry.
⋅ Post-implementation Review
Even after the change is finalized, periodic assessments are vital. These reviews monitor the change’s sustained effectiveness, ensuring it continues to meet the desired objectives without introducing unforeseen issues.
⋅ Feedback Collection
Gathering feedback from various departments and stakeholders provides insights into the change’s real-world impact, offering valuable lessons for future change control initiatives.
⋅ Continuous Improvement
The insights from these reviews feed into a cycle of continuous improvement, enabling better handling of subsequent changes and refining the change control process itself.
In essence, while closure marks the end of a particular change’s journey, the review ensures its long-term success and informs the approach to future change control endeavors.
With each step of the change control pharmaceutical process outlined, let’s take a look at visual change control flowchart in pharma.
Change Control Flowchart in Pharma
If you prefer to visualize the process, you’ll love our change control flowchart in pharma.
A change control flowchart in pharma is a visual tool depicting the steps in the pharmaceutical change control process.
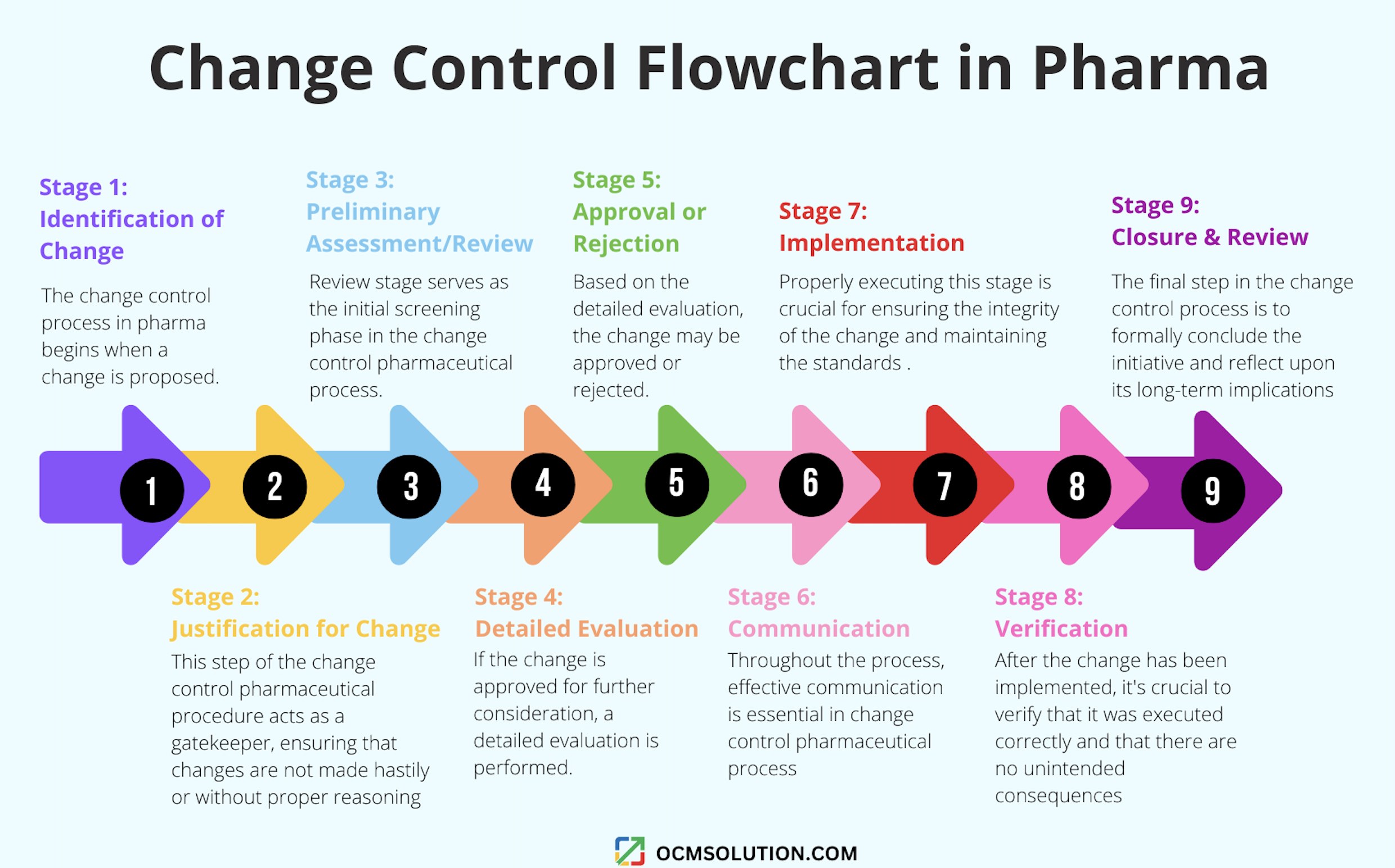 Change Control Flowchart in Pharma Industry
Change Control Flowchart in Pharma Industry
How can I utilize a change control flowchart in pharma?
⋅ Clarifying the Process
It outlines each step, simplifying complex procedures and showing the flow.
⋅ Training Tool
New employees can quickly grasp the change control pharmaceutical process, making onboarding efficient.
⋅ Facilitating Communication
It ensures alignment across departments, from R&D to Quality Assurance, by providing a unified visual guide.
⋅ Ensuring Compliance
It serves as a structured document during regulatory audits, showcasing a systematic change control approach.
⋅ Quick Reference
Any confusion during the change management in pharmaceutical industry can be resolved by referring to the flowchart.
To put it simply, flowchart streamlines, communicates, and ensures compliance in the change control management in pharmaceutical industry.
Importance of Change Management in Pharmaceutical Industry
Change management is pivotal in the pharmaceutical sector, and the significance stems from multiple facets:
- Compliance: The guidelines for change control in pharmaceuticals ensure products retain efficacy and safety. Following these guidelines is imperative for regulatory adherence.
- Product Quality: By applying the change control procedure in pharma, modifications—from raw materials to processes—are assessed to safeguard product integrity.
- Risk Management: An example of change control in pharma might be a supplier switch. By rigorously implementing the change control procedure in pharma, potential risks are anticipated and mitigated.
- Operational Excellence: Examples of change control in pharma, like process alterations, can boost efficiency and cost-effectiveness when managed correctly.
In essence, the guidelines for change control in pharmaceuticals and standard procedures ensure patient safety, product quality, and regulatory compliance.
Are you looking for an example of change control form in pharma? Get in touch and let us know!
FREE Change Control in Pharmaceutical Industry PPT
Our FREE Change Control in Pharmaceutical Industry PPT presentation provides a comprehensive overview of the change control process within the pharmaceutical industry.
This useful change control in pharma PPT contains carefully designed slides that detail the flow and outline each stage of this crucial procedure.
From initial identification of changes to the final approval and documentation, the change control in pharma PPT presentation offers a step-by-step guide to ensure that quality and compliance are maintained at all times.
Download FREE Change Control in Pharmaceutical Industry PPT
While our change control in pharma PPT is designed to serve as a standalone presentation, you can also modify and adjust it to better suit your individual needs or organizational practices.
Whether you’re using it for presentations or internal communications, this tool offers a solid foundation on the subject.
Don’t Miss: Best Step-by-Step Guide to Change Readiness Assessment
Conclusion: Change Control Guidelines in Pharma
Change control in the pharmaceutical industry stands as a testament to the sector’s commitment to quality, safety, and regulatory compliance. Through meticulous processes and evaluations, it ensures that any alterations to existing protocols, products, or systems enhance patient well-being and meet stringent standards.
As the industry evolves and new challenges emerge, robust change control mechanisms will remain pivotal in maintaining trust, upholding integrity, and ensuring the continued delivery of effective and safe medicines to the global populace.
It’s worth noting that regulatory bodies, like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have change control guidelines in pharma.
Non-compliance can result in regulatory actions, recalls, or even facility shutdowns. Therefore, maintaining a robust change control pharmaceutical process is critical for manufacturers in this industry.
FAQ: Quick Overview of Change Control in Pharma
What is definition of change control in pharma?
Definition of change control in pharma refers to a systematic approach employed within the pharmaceutical industry to manage and document any modifications to established processes, systems, or products. This ensures the consistent production of high-quality medicinal products, even in the face of alterations.
What are the stages of change control procedure in pharma?
Stages of change control in pharma are:
• Identification of Change
• Justification for Change
• Preliminary Assessment/Review
• Detailed Evaluation
• Approval or Rejection
• Communication
• Implementation
• Verification
• Closure and Review
What is change control SOP in pharma?
The Standard Operating Procedure (SOP) for change control delineates the structured change control process in pharma, providing a clear roadmap for ensuring consistency, traceability, and compliance.
What is change control format in pharma?
In the pharmaceutical industry, the change control format refers to a standardized template or form used to document the entire change control process.
Note: Content on OCM Solution's ocmsolution.com website is protected by copyright. Should you have any questions or comments regarding this OCM Solution page, please reach out to Ogbe Airiodion (Change Management Lead) or the OCM Solutions Team today. OCM Solution was previously known as Airiodion Global Services (AGS).
External sources: stock.adobe.com